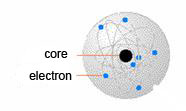
Rutherford's model
Rutherford designed the atom like the solar system. In this model the electrons move around the nucleus just like the planets around the sun.
Rutherford's model was the first successful model for the atom. It was designed after the famous Rutherford's experiment in which Rutherford discovered that almost all the mass of the atom is in the center (in the nucleus). The rest of the atom is empty space, and the electrons (which are responsible for the chemical properties of the atom) move around the nucleus.
Later experiments led Bohr to design a new model (Bohr's model).
Even newer experiments led Schrodinger to design an even newer model, (Schrodinger's model).